Sodium-Iodide Symporter
Turning research discoveries into clinical therapies
Through Mayo Clinic’s Employee Entrepreneur Program (a program that provides support and resources for medical innovators), researchers Kah Whye Peng, Ph.D. and Stephen J. Russell, M.D, Ph.D., commercialized a sodium-iodide symporter (NIS) reporter gene imaging system and subsequently created a company called Imanis Life Sciences in 2012.
Fast forward to 2019 when they launched their second venture, Vyriad, which is a clinical-stage company developing oncolytic virus therapies for the treatment of cancers. Vyriad’s innovative approach relies on viruses (developed in labs at Mayo Clinic and the University of Miami) that are engineered to destroy cancer cells directly while kick-starting and assisting the body’s natural tumor-killing immune response. They use the NIS system to track these engineered viruses.

“So now, instead of putting your therapy into this black box and just hoping for a favorable outcome, now the box is clear glass, and there’s a light on. You can see what’s happening.” Kah Whye Peng, Ph.D.
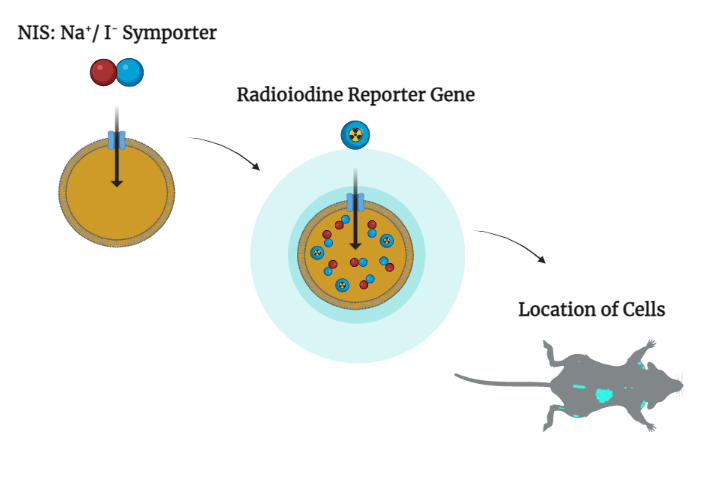
Turning the light on
Imaging technology begins with something called a reporter gene. In Dr. Peng’s system, a human protein called sodium-iodide symporter, or NIS, fulfills this role. Iodine — or radioactive iodine tracer — can accumulate in tissues if the cells there have been engineered to express NIS. While light from fluorescent and luminescent reporters is too weak to shine through layers of tissue, the electromagnetic radiation arising from iodine tracers penetrates readily.
“The technology is very simple, but can be applied across many applications, from regenerating a liver or regenerating bone marrow to following (cancer-killing) viral therapy,” explains Dr. Peng. “Whatever the therapy, you need to know its fate.”
Delivering helpful genes to cells
In research on some types of malignancies, including ovarian cancer and multiple myeloma, therapeutic viruses find, infect and kill cancer cells, and then stimulate an immune response so that patients can fight any cancer that tries to grow back. When any type of engineered cell or virus is released into the body, there are questions to be asked, such as has it reached the treatment site, or is it selectively propagating and otherwise doing what it’s supposed to?
If the beacon is in place, a patient could receive a small dose of radiotracer. After an hour, the tracer accumulates in any cells expressing the DNA fixes. After being scanned from nose to toes, the patient can go home and drink plenty of water to flush out the radioactivity, which has a quick half-life. And at their next visit, the patient can view a 3D representation of the treatment on screen.

Boosting the body's natural ability to heal
Typically, gene and cell therapies deliver DNA to diseased tissues or organs, where they can restore function. But regenerative medicine also can be used to boost the body’s natural ability to mend itself — to remodel cells and tissues, and promote self-healing processes. The liver has the greatest regenerative capacity of any organ in the body, and Mayo Clinic researchers are developing a number of regenerative therapies for patients who today must wait for whole-liver transplants. Dr. Peng and her colleagues, including Raymond Hickey, Ph.D.; Scott Nyberg, M.D., Ph.D., a Mayo Clinic transplant surgeon; and others were the first to demonstrate the value of NIS reporter gene technology in liver regeneration — 3D imaging liver cell engraftment in individual animals over time in a way that is relevant to human clinical settings.
Turning research discoveries into clinical therapies
Regenerative therapies are pharmaceuticals, and their kinetics — movements and activities — within the body must be tracked and fully understood before they can be used in patients.
“The noninvasive monitoring of cell fate is critical,” explains Dr. Peng.
Twenty years and counting into her career at Mayo Clinic, Dr. Peng is now participant and witness as regenerative therapies wend their ways from discovery to clinical trials and onward toward Food and Drug Administration approval.
Share this story
A version of the story was originally published in Mayo Clinic’s Discovery Edge.
Mayo Clinic and Drs. Peng and Russell have a financial interest related to the NIS imaging technology referenced in this article. Mayo Clinic will use any revenue it receives to support its not-for-profit mission in patient care, education and research.
